
- CHEMISTRY CALCULATOR BALANCE EQUATION HOW TO
- CHEMISTRY CALCULATOR BALANCE EQUATION PLUS
- CHEMISTRY CALCULATOR BALANCE EQUATION FREE
CHEMISTRY CALCULATOR BALANCE EQUATION HOW TO
How to Balance Equations Chemistry?Īs you already know chemical equation balancing can be done if reactants and products’ atoms are brought the same in number. Now to comprehend with the chemistry involved in this reaction and to balance the reaction, you must make use of the balance equations calculator chemistry.
CHEMISTRY CALCULATOR BALANCE EQUATION PLUS
Each substance in a chemical reaction is separated by a plus sign (+). “The chemical equation is defined as the symbolic representation of a chemical reaction, with reactants on the left and products on the right.”Īrrows separate reactants and products. Let’s move on! What Is Chemical Equation? So if you are indulging with complex reactions, no need to be worried more! This chemical equation calculator with states balances different equations of chemical reactions.Ĭontinue reading to learn how to balance chemical equations either manually or by using this best chemical equation balancer. There are many sample equations in this chemical equation balance calculator so that you can practice and balance equations.
CHEMISTRY CALCULATOR BALANCE EQUATION FREE
I would love to know which of the three methods you prefer.Use this free online balancing chemical equations calculator to balance any equation, structure, and find equilibrium constant with chemical names and formulas. So, now that you’ve gone through the basics, how about testing out your skills? Each level below has 5 questions. I’ll walk you through the 4 steps involved in this video: Basically, what you’ll do is set up a bunch of equations and then solve them to get the coefficients (numbers in front of the formula). The final method is for those who loves solving algebraic equations or when you’re trying to balance complex equations (like the ones in Ninja level – see practice questions below). There are 4 simple steps in balancing equation using this method. It pretty much works like the first method, however, you keep track of the changes by writing them down. We’ll try out the second method on this new equation. You can take my word for it, or give it a try and see how long it’ll take you to balance this equation by inspection.
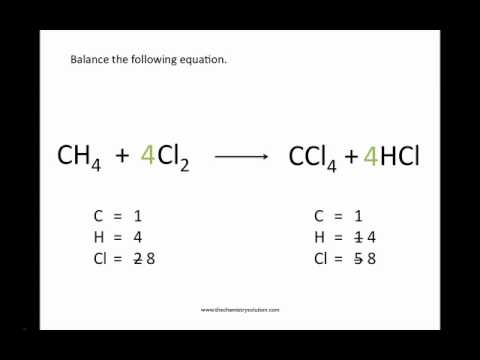
However, it gets taxing when you encounter more complicated equation like this: C 6H 5F + O 2 → CO + H 2O + F 2. This is a rather popular method and it works pretty well to a certain extent. Since we added a 2 in front of NaCl, our Na on the right-hand side is no longer balanced, so we’ll need to add a 2 in front of NaF as well. So, we’ll place a 2 in front of NaCl like this: Looks like it’s not balanced since there’s one on the left but two on the right. Na is balanced since there is one on the left-hand side and one on the right-hand side. You balance the equation by inspecting it. Personally, I prefer Method 2 when it comes to solving easy to intermediate equations because it is simple and doesn’t tax my brain too much. There are 3 methods in mastering this art. If you count the number of atoms on both sides, you’ll notice that they are not equal: The compounds on the left-hand side (before the arrow) are called reactants while the compounds on the right-hand side (after the arrow) are called products. So the main goal of balancing chemical equation is to get the number of atoms to be the same on each side of the equation. You’ll be able to get the molar ratios for all the compounds involved in the reaction and that will enable you to perform various types of calculations correctly (more on that in future posts). Jokes aside, being able to balance chemical equation will open up a lot of possibilities. Is it important to be able to balance chemical equations correctly and quickly? You bet! Why so? First of all, you’ll make your chemistry teacher happy and maybe impress someone else along the way.


 0 kommentar(er)
0 kommentar(er)
